Roadmap to a Successful Manufacturing Transfer
By Alan Sundheimer, VP of Development and Technology
PMC SMART Solutions has deep roots in the medical industry, including engineering, design/development, and plastic molding and contract assembly, and extensive experience with managing product design and manufacturing transfers. We believe that transfers will continue to increase in response to supply chain difficulties our own OEM customers are experiencing. It is with this in mind that we present this white paper to offer some insights regarding supply chain development and management and how to avoid the risks that are commonly associated with transferring or moving production lines from one supplier/location to another.
In a competitive, complex global manufacturing climate that is further complicated by a pandemic, one can expect to encounter issues that challenge the supply chain status quo. This becomes particularly apparent as medical OEM companies look to expand and transfer progressively more product design and manufacturing programs to keep up with a demanding healthcare market. Much has been learned about COVID-19, but no one is certain how it will ultimately affect suppliers’ ability to continue supporting medical OEM customers and their inherently stringent requirements.
Risk Factors and Stakeholders
While product development projects can be complex, one can start from scratch and navigate the direction of the design and manufacturing processes. Conversely, transfers of existing product lines have their own set of complexities, and there is a lot at stake, especially if a product is already being sold in the marketplace. For example, with an existing program transfer, if an inherent design issue is discovered, the ability to make necessary changes is often not a simple undertaking. There is little, if any, opportunity to move away from the existing design and manufacturing methods to minimize risk, so it becomes more of a risk management project. One must think critically to find manufacturing processes and “smart fixes” that will enable continued production of a device that is of the same or better quality as it was previously.
Additionally, production can be adversely affected by supply chain interruptions during a project transfer. For instance, managing one’s supply chain during a transfer entails building (or rebuilding) product inventory, working with new suppliers to onboard secondary suppliers, the possible transfer of critical equipment and tools, revalidation of production lines and processes and dealing with inherent hiccups and risks in these activities. If these activities are not managed well, it can cause delays or even stoppages in the delivery of critical medical supplies to the marketplace. This impacts not only the OEM’s production goals but also the medical industry’s ability to serve its customers. All are stakeholders, even if downstream, because patients who need procedures that require use of a product that is being transferred to a new production facility may experience delays or cancellations.
One must also take into consideration the OEM’s manufacturing engineers, quality managers, supply chain/purchasing personnel, who are all stakeholders in the successful transfer of design and manufacturing work. Manufacturing engineers need a manufacturing partner that will work continually on operational excellence, seek ways to improve processes and reduce costs. Quality managers must ensure that industry standards are being met or exceeded throughout the manufacturing process by working with a manufacturing partner that can help them avoid or resolve issues while at the same time speeding products to market. OEM supply chain/purchasing personnel oversee the consolidation of business to strategic suppliers, and in so doing form the foundation of a company’s supply chain, so they must have partners they can rely on and trust. None of these stakeholders, nor their suppliers, can work independently of each other. If they do, a project transfer will surely experience issues.
Rebuilding Supply Chain Partnerships
When considering transferring design and manufacturing programs it is incumbent upon the OEM team to take the opportunity to work with a manufacturing partner that has the expertise to objectively evaluate its current supply chain with an eye on improvement. It is not uncommon to find that one’s supply chain is fragmented with too many supplier companies including those that may have been inherited from previous leadership. Is the status quo meeting needs production-wise and financially? Keep in mind that the number of suppliers directly drives overhead costs.
Partnering with a company that has broad experience with supply chain development and management that understands and is equipped to execute transfer work requirements and solutions will ensure a successful and unbiased evaluation and, as a result, a smooth transfer. Not all companies do this well. For example, larger suppliers may offer some of the most advanced machinery and expertise, but they also carry a lot of overhead. The bottom line often trumps the overall care and attention given to the transfer process, leaving some customers cold. Conversely, small “mom and pop” shops may provide that kid-glove treatment and have every intention of serving the customer’s every need, but many have limited resources to respond quickly to potential challenges. They can be a risk during an economic downturn or pandemic, and they do not always stay up to date with FDA and regulatory mandates for critical products like medical devices.
Tips for Transfer Success
Following are some smart steps and tips OEMs can use to ensure that its transfer of a medical product production line is handled effectively.
Transparency to Build Trust
If a supplier says “yes” to all an OEM’s requests but does not provide a structured framework that supports the resolution of those requests, a red flag should go up. When assessing a transfer, having a specific process in place makes the transition much easier and reduces risk. A good supplier will keep the OEM customer informed of all issues occurring with the current production protocols and will call out any anticipated pitfalls that can occur before the transfer is even performed. Issues, concerns and suggested improvements must be discussed openly to ensure a more seamless and successful transfer.
An Eye on Improvement
Look for a vendor that actively identifies opportunities for improvement and has a wide range of manufacturing capabilities. The vendor should be able to onboard existing suppliers and evaluate alternate suppliers with new technologies that can help reduce costs. Specifically, the vendor should have a strong supply chain portfolio and a management team that can perform an in-depth analysis of the program and rebuild a supply chain or replace problematic suppliers to ensure a smooth transfer.
Look for Responsiveness!
In addition to having the capability to make improvements to a program transfer’s supply chain, it is also important for a vendor to be nimble and quick to execute those improvements. Ask whether the vendor has a dedicated, cross-functional product team that understands time-to-market urgency and can get the job done right. Is the company up to date on medical manufacturing regulations, FDA registered and ISO 13485 certified? Having this information up front can be instrumental in avoiding issues later.
Cost Considerations
Time to market is extremely important, but so is keeping costs in check. A good vendor keeps both interests in view knowing it is possible to leverage economies of scale with a smaller, more effective supply chain and will look for cost-saving opportunities that the OEM customer may not have the time and resources to identify. In addition, it is just easier to manage fewer suppliers.
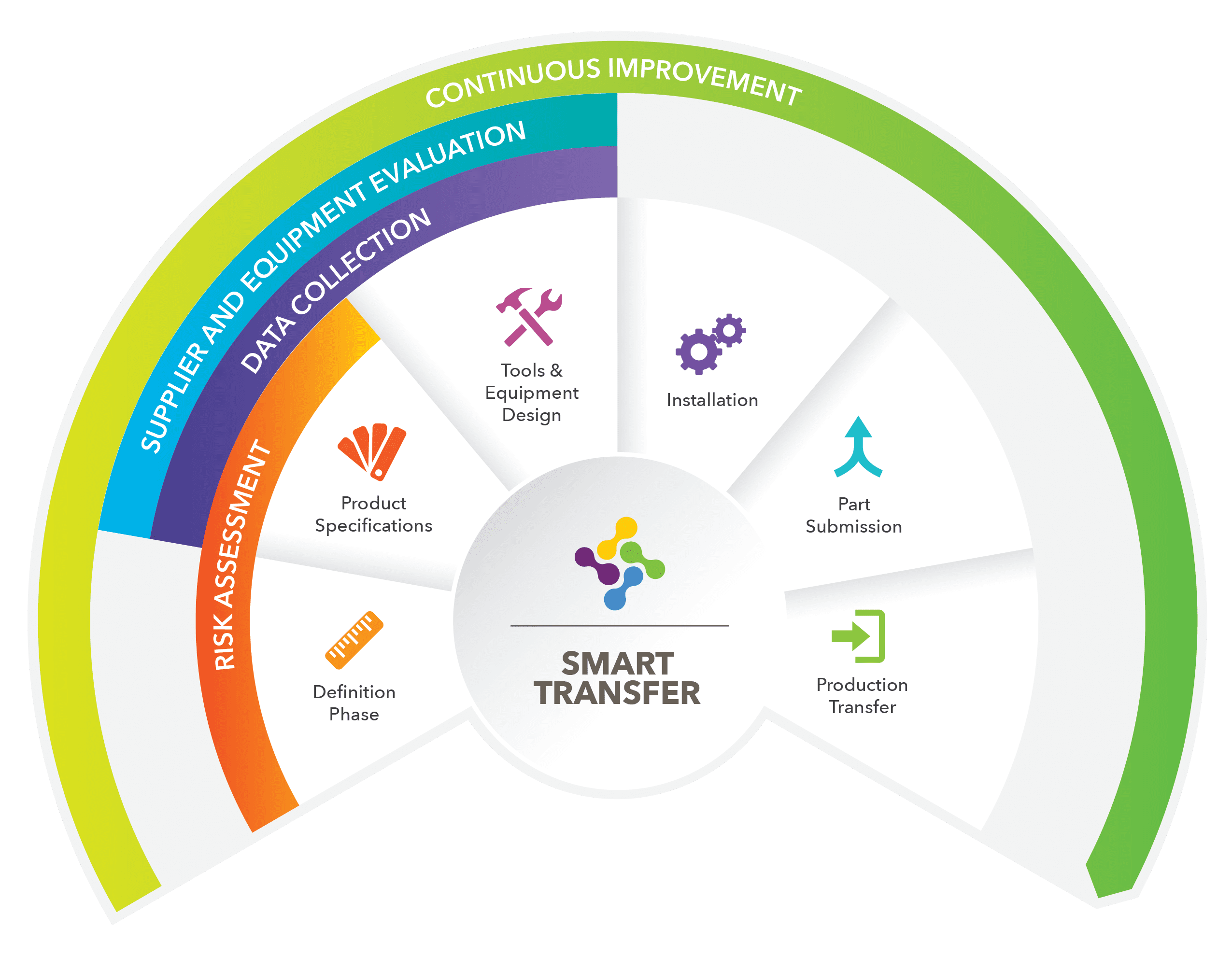
In summary, PMC SMART Solutions utilizes a well-established transfer process called SMART Transfer, that is integrated within our quality system. The SMART Transfer process ensures world-class, reliable results for any program, large or small. As a result:
If you are interested in learning more about recent PMC SMART transfer programs, please contact your PMC representative, contact us by visiting our website or give us a call at 513-921-5040.